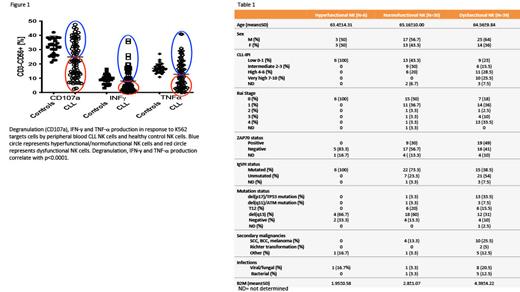
Chronic lymphocytic leukemia (CLL) is a heterogeneous disease, presenting with a highly variable clinical course, outcome and response to therapy. This variability has been linked to a complex tumor microenvironment and genetic/epigenetic modifications that lead to immunosuppression. Indeed, the clinical hallmark of this disease is the susceptibility to infections and secondary malignancies. While T cell dysfunction in CLL has been well described, conflicting information exists on NK cell changes in CLL and their role in CLL immunosurveillance. Here, we analyzed samples from 75 untreated (or completed treatment >2 years ago) CLL patients and 30 healthy controls. We discovered a substantial variation in NK cell function compared with healthy controls, which we further classified into hyperfunctional, normofunctional and dysfunctional based on their ability to degranulate, and produce IFN- γ in response to K562 targets ( Figure 1). NK cells from ‘dysfunctional’ CLL samples also displayed impaired cytotoxicity against K562 targets, weaker synapse formation and Syk/Zap70 signaling in response to antibody-dependent cellular cytotoxicity (ADCC) compared to those from ‘functional’ samples. The NK functional status was strongly associated with multiple prognostic markers, including cytogenetics, IgVH mutation status, ZAP-70 expression and disease stage, with patients with dysfunctional NK cells having significantly higher rates of secondary malignancies and viral infections ( Table 1). Using multispectral flow cytometry we discovered high phenotypic heterogeneity with unique NK cell clusters present in the dysfunctional samples. Those clusters express a pattern of activation-induced exhaustion, with upregulation of multiple checkpoint molecules such as PD-1, TIGIT, LAG-3, KLRG-1, TIM-3 but not evidence of terminal differentiation (CD57 negative). Those clusters also showed concomitant expression of activating molecules such as NKG2D and DNAM-1, suggesting reversibility of their dysfunctional status. We subsequently found significantly higher (p<0.0001) constitutive phosphorylation of SHP-1 protein, a phosphatase downstream of multiple inhibitory NK cell receptors, in NK cells from dysfunctional samples. Treatment with a SHP-1 inhibitor NSC-87877 (EMD Millipore) or use of siRNA targeting SHP-1 (ThermoFisher scientific), resulted in reversal of NK dysfunction with full restoration of their functional capacity. Taken together, our data suggest that NK cells functional status plays an important role in CLL disease progression and secondary complications. The novel mechanism of NK cell immune dysfunction in CLL described here has the potential to improve outcomes with novel immunotherapies such as chimeric antigen receptors, checkpoint inhibitors and bi-specific antibody therapy that have shown disappointing results in CLL to date when compared with other B cell malignancies.
Disclosures
Shaim:Takeda: Patents & Royalties. Rafei:Takeda: Other: H. R. and The University of Texas MD Anderson Cancer Center have an institutional financial conflict of interest with Takeda Pharmaceutical. . Basar:Takeda, Affimed GmbH: Patents & Royalties. Daher, MD:Takeda: Patents & Royalties. Banerjee:Takeda: Patents & Royalties, Research Funding. Kerbauy:Takeda: Patents & Royalties. Lin:Takeda: Patents & Royalties, Research Funding. Uprety:Takeda: Other: N.U. and The University of Texas MD Anderson Cancer Center have an institutional financial conflict of interest with Takeda Pharmaceutical. . Acharya:Takeda: Other: S. A. and The University of Texas MD Anderson Cancer Center have an institutional financial conflict of interest with Takeda Pharmaceutical. . Liu:Takeda: Patents & Royalties. Ang:Takeda: Patents & Royalties. Burger:Janssen: Other: Speaker fees and Travel Support; AstraZeneca, Pharmacyclics: Other: Advisory Board, Research Funding; Abbvie, Beigene: Research Funding. Wierda:Gilead Sciences: Research Funding; Nurix THerapeutics: Research Funding; AstraZeneca/Acerta Pharma: Consultancy, Research Funding; Numab THerapeutics: Research Funding; Accutar Biotechnology: Research Funding; Bristol Myers Squibb (Juno & Celgene): Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; GSK/Novartis: Research Funding; National Comprehensive Cancer Network: Other: Nonrelevant Financial Relationship/Chair, CLL). Supported by the NIH/NCI under award number P30 CA016672 and used MDACC Cancer Center Support Grant (CCSG) shared resources; Juno Therapeutics: Research Funding; Janssens Biotech: Research Funding; NIH P30 CA016672/MDACC Cancer Center Support Grant: Research Funding; Genentech: Research Funding; Pharmacyclics LLC: Research Funding; KITE Pharma: Research Funding; Sunesis: Research Funding; Janssens Biotech Inc: Research Funding; GlaxoSmithKline: Research Funding; Loxo Oncology, Inc./Lilly: Research Funding; Cyclacel: Consultancy, Research Funding; Oncternal Therapeutics, Inc.: Research Funding; Miragen: Research Funding. Ferrajoli:Beigene: Research Funding; AstraZeneca: Honoraria, Research Funding; Janssen: Honoraria; GenMab: Research Funding; Genetech: Honoraria; Abbvie: Honoraria, Research Funding. Shpall:NY Blood Center: Membership on an entity's Board of Directors or advisory committees; Adaptimmune: Membership on an entity's Board of Directors or advisory committees; Navan: Membership on an entity's Board of Directors or advisory committees; Celaid Therapeutics: Membership on an entity's Board of Directors or advisory committees; Fibrobiologics: Membership on an entity's Board of Directors or advisory committees; Axio: Membership on an entity's Board of Directors or advisory committees; Takeda: Other: License agreement; Affimed: Other: License agreement; Syena: Other: License agreement. Rezvani:Affimed: Other: License agreement; Takeda: Patents & Royalties.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal